Test for covid-19 antibodies on dried blood spots (DBS)
One newly discovered mutant of the SARS-CoV-2 virus is omicron. The scientific community is working intensely to clarify if the available vaccines protect against omicron, and if the rapid test antigen for the spike 1 protein recognizes the virus and with what sensitivity. Antibodies produced due to omicron infection may also be different from other mutations and undetectable with IgG assays based on the original spike 1 protein.
Vitas has developed and validated an antibody (IgG) test for covid-19 in Dried Blood Spots (DBS) based on the spike 1 protein. The test measures IgG antibodies against all SARS-CoV-2 mutants prior to omicron produced as a consequence of natural virus infection or mRNA vaccines. The test can be bought in Norwegian pharmacies. We are currently investigating if our assay capture IgG produced after infection with omicron.
An update will follow when new information is available.
The test is commercially available to private persons through these partners:
Vitusapotek.no
Apotekdirekte.no
Bodymarkers.no
The reception at Oslo Science Park, Gaustadalléen 21, 0349 Oslo
You can find your results at: https://www.vitas-reports.no
For purchase of more than 25 kits, you can contact Vitas AS directly, pay by Vipps (Vitas AS, no. 133669) or ask for invoice, and we will send it directly to your preferred address.
If you represent an organization or company and would like screening of your staff, please contact us for an offer or discussion on how to use this product and related services.
If you would like to become a reseller of the Vitas covid-19 kit you can send an e-mail to Thomas Gundersen, teg@vitas.no.
The test
The test shows excellent performance (>94% sensitivity and 99.9 % specificity (n= 601). The test includes a CE IVD marked DBS collection kit, and a CE IVD marked ELISA assay using spike protein S1 as antigen. An extended validation in 1000 individuals was completed 1. July 2020. The purpose was to test the assay in a routine setting, get feedback on user friendliness, and investigate the rate of infection. 50 SARS-CoV-2 PCR positive volunteers have submitted DBS for IgG repeated measurements. From this we have calculated that the IgG antibody blood concentration is reduced by a half-life of approximately 110 days.
Our newly developed antibody assay on DBS has been used in two large Norwegian studies
The first one named “TRAIN” included 3400 subjects associated to SATS exercise centers in Oslo in June 2020. The purpose was to examine if there were more covid-19 infections among individuals training inside the centers as compared to those training outside the centers. The results showed that very few of the tested individuals were positive for covid-19 antibodies, indicating that very few had been infected, and there was no difference in percentage of positive samples among people training outside or inside the centers (link to the study).
The second study named “Corona and Immunity in Norway” included about 30 000 subjects, representative of the general adult Norwegian population 16 years of age and older. The study was carried out by scientists at University of Tromsø in December 2020 and January 2021. Enhanced concentration of covid-19 antibodies was highest among those aged 16-19 years (1.9%), those born outside the Nordic countries 1.4%, and in Oslo 1.7 %, and Vestland 1.4%. An article is in press with these results (Anda EE et al. Seroprevalence of antibodies against SARS-CoV-2 virus in the adult Norwegian population, winter 2020/2021: pre-vaccination period (link to the study).
Vaccines and the IgG DBS test
The test Vitas uses on the DBS platform detects IgG antibodies against the spike protein S1 critically important for the binding to human cells thereby allowing transfer into the cells and replication of virus. Most covid-19 vaccines are based on developing antibodies and T cells active against the S1 protein. Thus, the Vitas’ DBS covid-19 test represents a very good way to evaluate the response to most vaccines.
This type of DBS testing is very convenient in epidemies and turns out to be a suitable marker of immunity against covid-19.
Interpretation of the results
Arbitrary units (AU) are used in the results you get via our homepage: https://www.vitas-reports.no.
| AU | Short evaluation | Explanation |
|---|---|---|
| 0-0.8 | Negative | Probably no infection or vaccine |
| 0.81-1.1 | Borderline | Perhaps infected/Vaccinated but antibody level is marginal |
| > 1.1 | Positive | Probably infected/vaccinated |
| 2-6 | Positive | Normal range after 1. vaccine |
| 5-11 | Postitive | Normal range after 2. vaccine and booster |
Background
The world is still in the middle of what probably is the worst pandemic in many decades. Currently (December 2021), more than 260 million people have been infected and more than 5.2 million have died infected by the SARS-CoV-2 virus worldwide. To limit the extent of the infection, reduce the number of people in need of advanced hospital treatment, and to avoid overloading the health care system, Norwegian and international authorities have periodically "closed" many communities. This has led to an unprecedented financial crisis. Up to about 80% of infected people will have no or just small symptoms, millions of people have been or are in quarantine. Subjects who have been infected exhibit partial or complete immunity, and thus reduced potential for being infectious.
A person can be infectious from a few days up to about 3 weeks after being infected. Detection of infection is done after sampling in the upper respiratory tract using a gene technology method called polymerase chain reaction (PCR). After about 3 weeks, most people will no longer have virus in their airways, but covid-19 leaves behind a "biological trace" of antibodies to some proteins from the virus. About 7 days after infection, the body starts producing these antibodies against the virus as a defense against new infections.
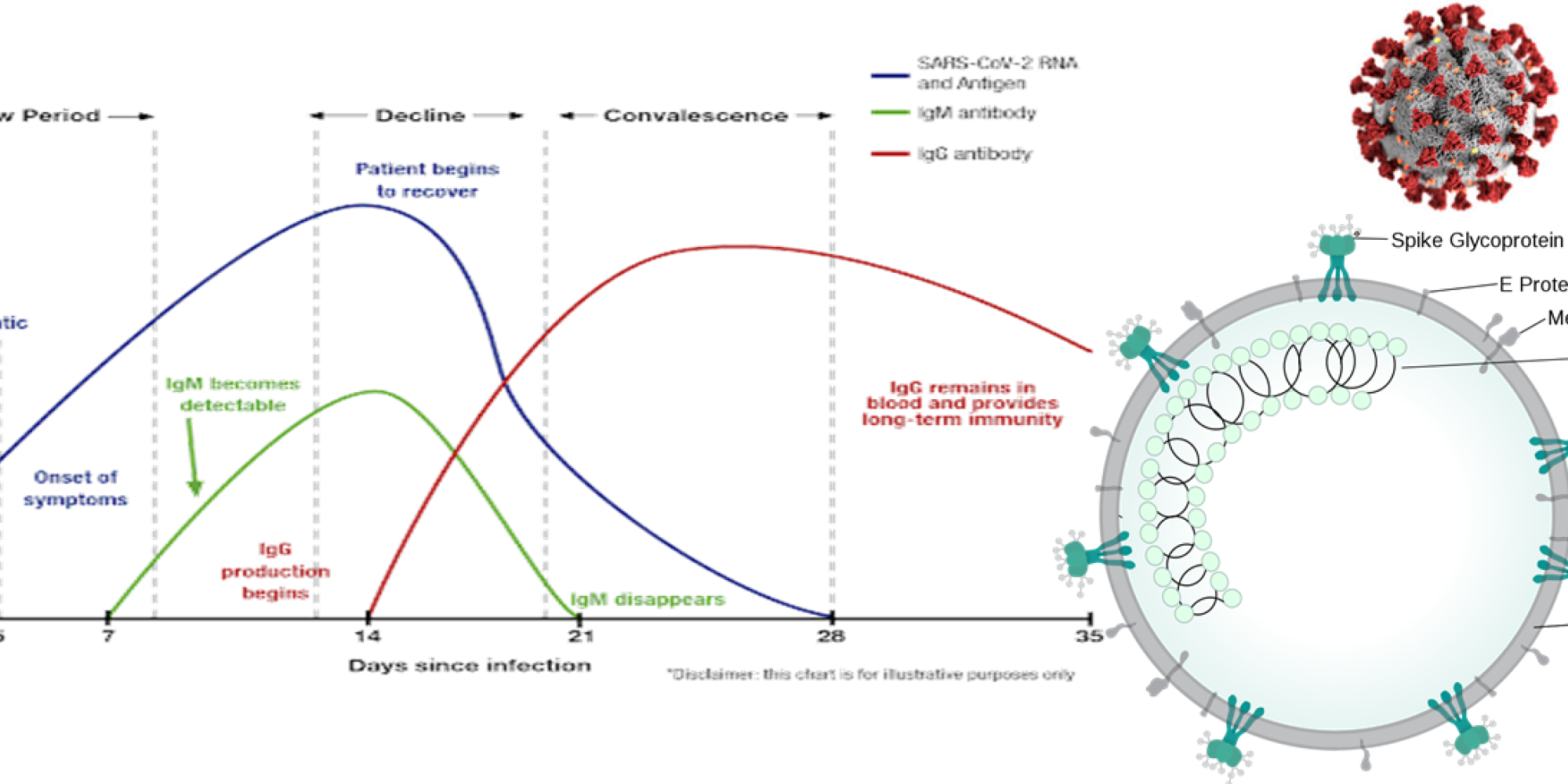
Detection of IgM and IgG antibodies against covid-19 in the blood is one way to identify individuals who may get out of quarantine, return to work, or get access to public actvities with reduced risk of infecting others or becoming sick again. However, mutants of the virus can behave differently concerning transmission and promotion of light or serious clinical disease. The new omicron mutant seems to be more infectious and perhaps promote less serious disease. Granted that mutations do not affect large areas of the spike protein 1, our present ELISA (enzyme-linked immunosorbent assay) can be used to detect natural infection as well as vaccine-induced antibodies.
Alternative sampling
It is common to use blood serum or plasma for these assays, which requires that health professionals take venous blood samples from the arm. It also requires centrifugation of the blood in a laboratory or medical office. As an alternative sampling Vitas has performed similar analyses from so-called Dried Blood Spots (DBS) for 15 years. A drop of blood from the fingertip is deposited on a special paper. The sample can be taken by the subject him/herself at home or at work without assistance of health professionals, and it can be sent by regular mail to the laboratory for analysis. This is especially important during epidemics.
The limited equipment required to take the blood sample is mailed to the subject or purchased at the grocery store or online. This type of distribution is very simple, inexpensive, and allows screening of large populations at low cost. The sensitivity of the test (subjects proven to have the disease and found positive by the test) is 94%. This means that out of 100 individuals, on average 6 will be negative for antibodies against SARS-CoV-2 although they have been infected. The specificity of the test (persons without covid-19 and with negative by the test) is close to 100%.
Mutants of SARS-CoV-2
Virus continuously evolve as their genetic code changes during replication (ref: CDC). Some of the most common mutants are listed below.
Alpha (B.1.1.7)
Beta (B.1.351)
Gamma (P.1)
Epsilon (B.1.427 & B.1.429)
Eta (B.1.525)
Iota (B.1.526)
Kappa (B.1.617.1)
1.617.3
Mu (B.1.621, B.1.621.1)
Zeta (P.2)
Delta (B.1.617.2)
Omicron (B.1.1.529)
In particular, the delta and omicron mutants have been in focus due to their quick spread in large populations. It seems as if both the delta and omicron mutants are more contagious as compared to the original Wuhan or alpha mutants. It is, however, not clear data on how the clinical disease is in relation to the delta and omicron mutants. The status at present (December 8, 2021) is that the mRNA vaccines with three dosages (two vaccines + booster) seem to be effective in producing neutralizing antibodies against omicron mutants of SARS-CoV-2 virus in vitro (in dishes in the laboratory (ref)). Moreover, preliminary data from studies on vaccination with three consecutive dosages suggest that a booster dose of BioNTech/Pfizer vaccine induces 75 % prevention symptomatic disease development (UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 31. 10 December 2021, pp 1- 42). However, due to the short observation times we have to await further information on this topic in the near future.
Covid-19 vaccines
Many institutions and pharmaceutical companies have invested heavily in developing effective vaccines against covid-19. More than 200 vaccine candidates at various stages of testing for a safe and effective vaccine have been described in New England Journal of Medicine August 26, 2020. Some countries and institutions collaborated to complete covid-19 vaccines quickly, testing efficacy and side-effects in phase III studies. Eight vaccine candidates were selected among the most promising vaccines based on 8 different manufacturers and four different biological principles on how to produce the vaccines.
Moderna and BioNTech/Pfizer are American and German/American biotech companies who developed a vaccine against covid-19 based on mRNA (messenger RNA) for the genetic code of one of the important surface proteins (spike 1) in the SARS-CoV-2 virus. The body's own cells are engaged to make the protein that the body also makes antibodies against, instead of injecting a virus protein itself directly into the body.
Two thorough articles with the first results from younger (18-55 years) and older (> 56 years) subjects show that antibodies are formed against the relevant virus protein, and the so-called type 1 Th (T helper) cells that can specifically react against the viral protein.
One of the important advantages of this mRNA vaccine is that it can be produced very quickly in large quantities without culturing the virus.
Moderna and other institutions/biotech companies tested several vaccines among 30,000 to 50,000 subjects in different population groups. The mRNA vaccines produced by BioNTech/Pfizer and Moderna were the first vaccine producers to get official approval, with some 90 % efficiency against transmission as well as development clinical signs, admission to hospitals, and death. The striking point is that the mRNA vaccines are very effective, and with few side-effects, although some cases of myocariditis (inflammation in the heart muscle) and pericarditis (inflammation in the thin sac surrounding the heart muscle) have occurred.
By developing the DBS-based test for antibodies against SARS-CoV-2, Vitas has taken part in the innovation required to reduce the burdens of the pandemic covid-19.
To learn more about our DBS analytical services, click here!