Test for Covid-19 on DBS
With support from the Norwegian Research Council, Vitas has developed and validated an IgG serology test for covid-19 in dried blood spots (DBS).
The test shows excellent performance (>94% sensitivity and 99.9 % specificity (n= 601). The test includes a CE IVD marked DBS collection kit as well as a CE IVD marked ELISA assay using spike protein S1 as antigen. An extended validation in 1000 individuals was completed 1. Juli 2020. The purpose was to test the assay in routine, get feedback from users on user friendliness and investigate the rate of infection among these 1000 subjects. 50 SARS-CoV-2 PCR positive volunteers are submitting DBS for IgG repeated measurements. From this we will learn at what rate IgG levels are reduced over time.
Upcoming vaccines and application of the IgG DBS test
The test Vitas has applied to the DBS platform detects IgG antibodies against the spike protein S1 critically important for the binding to human cells thereby allowing transfer into the cells and replication of virus. Most covid-19 vaccines are based on developing just antibodies against the S1 protein. Thus, the Vitas’ DBS covid-19 test is a very good way to evaluate the response to vaccines given to large populations. This type of testing will probably be essential as a marker of developed immunity against covid-19.
The test is commercially available to private persons through these selected partners.
You may visit the reception at Oslo Science Park at Gaustadalléen 21, 0349 Oslo, https://www.forskningsparken.no/ post@forskningsparken.no (+47 22 95 85 00) and buy them over the counter.
Bodymarkers AS at https://bodymarkers.com/ and purchase the test directly on-line from them.
Hjemmelegene www.hjemmelegene.no
Nettlegevakt: https://www.nettlegevakt.no/korona
You can find your results at: https://www.vitas-reports.no
In addition the test will be available through the below listed pharmacies:
| Pharmacy | Address |
|---|---|
| Ravnanger Apotek | Ravnanger senter, Kollevågveien 6, 5310 Hauglandshella |
| Drotningsvik Apotek | Drotninigsvik senter, Drotningsvikveien 130, 5179 Godvik |
| Apoteket Elgen | Vestlia 1, 1592 Folkestad |
| Ringen Apotek | Gjerdrumsvegen 5, 2040 Kløfta |
| Kløfta Son Apotek | Kolåsveien 4, 1555 Son |
| 123.Apotek | Rådhusveien 35a, 4640 Søgne |
| Torget/Torvet Apotek | Stasjonsgata 27, 3300 Hokksund |
| Lindeberg apotek | Jerikoveien 3, 1067 Oslo |
| Sunnfjord Førde Apotek | Naustdalsvegen 1A, 6800 Førde |
| Sunnfjord Florø Apotek | Hestenesgata 4, 6900 Florø |
| Sunnfjord Stryn Apotek | Tonningsgata 4, 6783 Stryn |
| Vettre Apotek | Konglungveien 206, 1392 Vettre |
For purchase of more than 25 kits, you can contact Vitas AS directly, pay by Vipps (Vitas AS, nr. 133669) or ask for invoice, and we will send it directly to your preferred address.
If you represent an organization or company and would like a screening of your staff, please contact us for an offer or discussion on how to use this product and related services.
If you would like to become a reseller of the Vitas Covid-19 kit you can send e-mail to Thomas Gundersen, teg@vitas.no.
Background
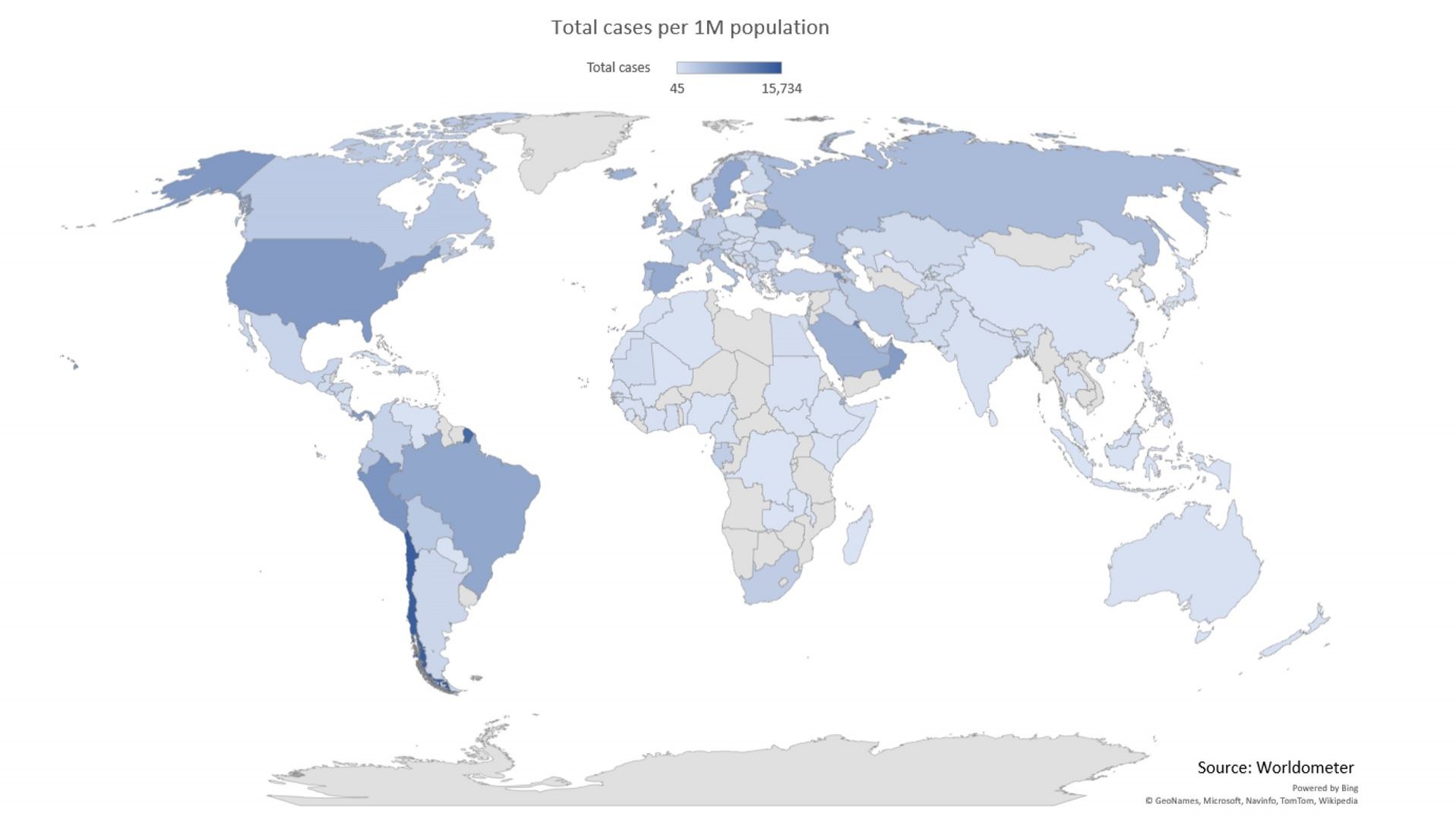
The world is in the middle of what probably is the worst pandemic in many decades. Currently (October 2020), more than 35 million people have been infected and more than 1 million have died infected by the SARS-CoV-2 virus worldwide. To limit the extent of the infection, reduce the number of people in need of advanced hospital treatment, and to avoid overloading the health care system, Norwegian and international authorities have "closed" many communities. This has led to an unprecedented financial crisis. Still, there are some places with limited capacity for monitoring virus infection with SARS-CoV-2. Due to the fact that up to about 80% of infected people will have no or just small symptoms, millions of people are in quarantine. Subjects who have been infected, probably exhibit partial or complete immunity, and thus may no longer be infectious. However, more data are required to conclude on this specific topic.
A person can be infectious from a few days up to about 3 weeks after being infected. Detection of infection is done after sampling in the upper respiratory tract using a gene technology method called polymerase chain reaction (PCR). After about 3 weeks, most people will no longer have virus in their airways, but covid-19 leaves behind a "biological trace" of antibodies to some proteins from the virus. About 7 days after infection, the body starts producing these antibodies against the virus as a defense against new infections.

These antibodies are proteins (immunoglobulins, Ig) found in the blood. First, immunoglobulin M (IgM) is formed and then immunoglobulin G (IgG). These antibodies in the blood can provide full or partial protection (immunity) against new infections with the SARS-CoV-2 virus. Thus, detection of IgM and IgG antibodies against covid-19 in the blood can be one way to identify individuals who may get out of quarantine and return to work with reduced risk of infecting others or becomming sick again. However, more data are required to conclude on this. The assays of antibodies against SARS-CoV-2 virus can be performed using ELISA (enzyme-linked immunosorbent assay) techniques.
It is common to use blood serum or plasma for these assays, which requires that health professionals take venous blood samples from the arm. It also requires centrifugation of the blood in a laboratory or medical office. As an alternative sampling Vitas has been performing similar analyses from so-called Dried Blood Spots (DBS) for 15 years. A drop of blood from the fingertip is deposited on a special paper. The sample can be taken by the subject him/herself at home or at work without a health professional, and it can be sent by regular mail to the laboratory for analysis.
The limited equipment required to take the blood sample is mailed to the subject or purchased at the grocery store or online. This type of distribution is very simple, inexpensive, and allows screening of large populations. The sensitivity of the test, ie subjects proven to have the disease and are found positive by the test is 94%. This means that out of 100 individuals, on average 6 will be negative for antibodies against SARS-CoV-2 although they have been infected. The specificity of the test, ie persons who have proven not to have had covid-19 disease and who are found to be negative by the test, are expected to be close to 100%.
Covid-19 vaccines
Many institutions and pharmaceutical companies have invested heavily in developing effective vaccines against covid-19. There are more than 200 vaccine candidates at various stages of testing for a safe and effective vaccine as shown in an article in the highly trusted journal New England Journal of Medicine August 26, 2020 provides an overview of how some countries and institutions are collaborating to complete covid-19 vaccines as quickly as possible, testing efficacy and side-effects in phase III in 2020. Eight different vaccine candidates were selected among the most promising vaccines based on 8 different manufacturers and four different biological principles on how to produce the vaccines. In addition, it should be possible to produce the vaccines in many million dosages when they are completed during the first half of 2021.
Moderna is an American biotech company focused on developing a vaccine against covid-19 based on mRNA (messenger RNA) for the genetic code of one of the important surface proteins in the SARS-CoV-2 virus. The body's own cells are engaged to make the protein that the body also makes antibodies against, instead of injecting a virus protein itself directly into the body.
Two thorough articles with the first results from younger (18-55 years) and older (> 56 years) subjects show that antibodies are formed against the relevant virus protein, and the so-called type 1 Th (T helper) cells that can specifically react against the viral protein.
One of the important advantages of this mRNA vaccine is that it can be produced very quickly in large quantities without culturing the virus.
Moderna and other institutions/biotech companies are now in phase III with testing of several vaccines among 30,000 to 50,000 subjects in different population groups. The hope is to have a vaccine ready for use during the first half of 2021.
Who gets the vaccine first?
After the vaccines have been developed, tested and produced, vaccination will be offered to healthcare professionals and certain risk groups like old people (< 65 years) and with conditions enhancing their risk of getting seriously ill.
Norway has secured the opportunity to purchase vaccines for the Norwegian population, and for a fair distribution of vaccines to low- and middle-income countries as well.
By developing the DBS-based test for antibodies against SARS-CoV-2, Vitas has taken part in the innovation required to reduce the burdens of the pandemic covid-19.
To learn more about our DBS analytical services, click here!